The development of sustainable and bio-inspired materials is driving innovation in rare-earth element applications. The study described here successfully implemented high-density peptide microarrays and TOF-SIMS to identify selective peptide hosts for europium ions. This opens new possibilities for designing bio-based materials with controlled properties with implications for sensors, catalysts, and diagnostic devices.
Background
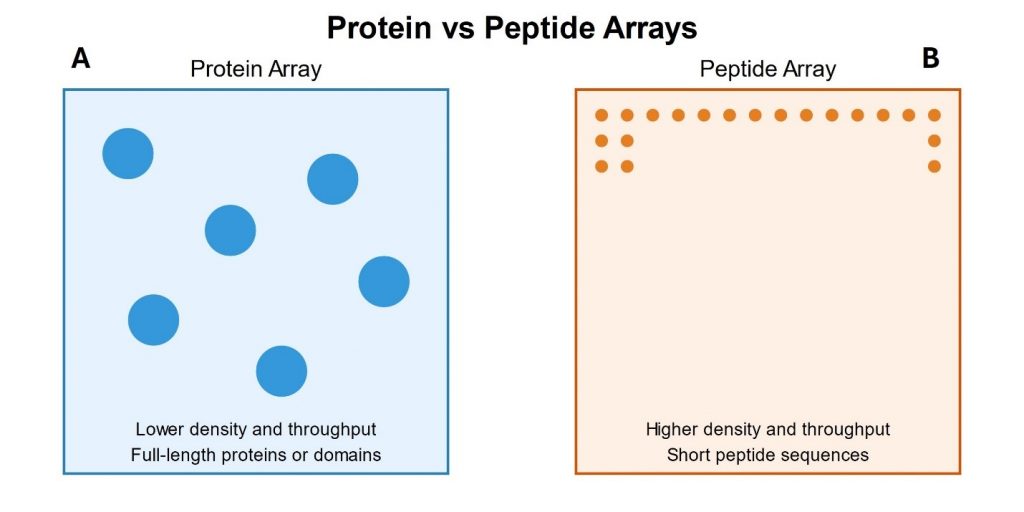
The materials science field is currently experiencing two major trends: a shift towards bio-inspired materials and the reduction of harmful substances. Peptides and proteins have emerged as promising host materials for rare-earth ions owing to their unique structural properties and versatility [1]. Their molecular framework provides natural binding sites through amino acid side chains, whereas their self-assembly enables the formation of complex structures such as nanotubes and nanodiscs. These characteristics, combined with the precision of modern synthesis techniques, make peptides ideal candidates for hosting rare-earth ions, potentially leading to applications in nanotechnology, catalysis, and biotechnology [2-4].
Recent work [1] has demonstrated the potential of lanthanide-binding peptides as molecular sensors and functional materials. Building on this foundation, Madirov and collaborators [5] developed a systematic screening approach using high-density peptide arrays to identify and characterize selective peptide hosts for europium ions.
Methodology
- The authors created a peptide library containing 10,000 different twelve-mer peptides, synthesized on a chip with 120 μm spots, based on earlier work with lanthanide-binding peptides [1].
- The library design was comprehensive, including substitution variants of a previously reported peptide and random combinations of positively charged and hydrophobic amino acids.
- Initial screening involved incubating the peptide array with europium nitrate salt solution at a concentration of 100 µg/mL for three hours.
- The authors initially detected europium ion deposition using a hyperspectral camera. However, preliminary tests revealed that hyperspectral imaging was effective only at concentrations above 5 mg/mL.
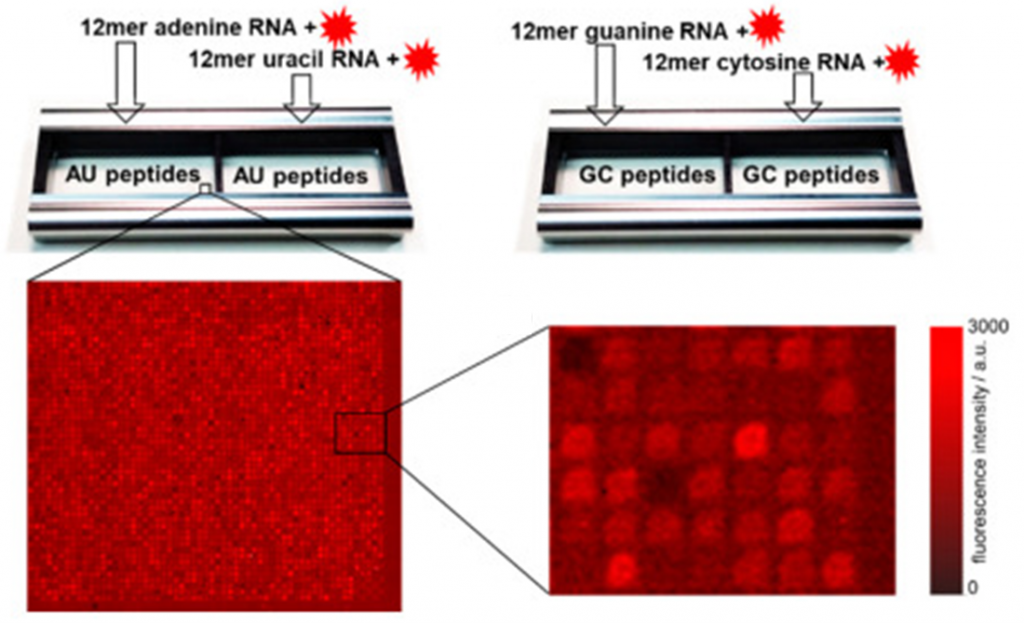
- Due to the need for superior chemical selectivity, spatial resolution, and precise detection of peptide components and bound europium ions [6,7], the team shifted to Time-of-Flight Secondary Ion Mass Spectrometry for Molecular Imaging (TOF-SIMS) analysis.
Key Findings
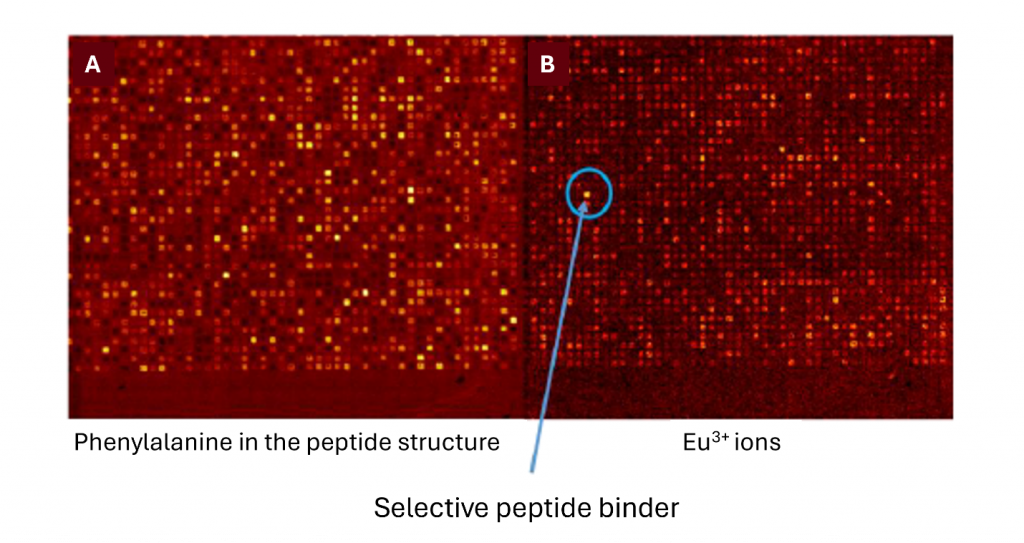
- The study successfully demonstrated the detection of selective europium ion deposition on different peptide sequences using TOF-SIMS imaging. The high chemical selectivity and surface sensitivity of this technique provided precise detection of peptide components and bound europium ions (Figure 1).
- Phenylalanine, an amino acid known for its role in forming ordered structures through its aromatic side chain, was clearly detected in the peptide array. This observation confirmed the ability of TOF-SIMS imaging to identify specific amino acids and their potential role in rare-earth ion binding.
- Europium ion screening revealed varying binding strengths across different peptide sequences, with the strongest interactions showing a signal-to-noise ratio over ten. This variation in binding strength suggests the possibility of fine-tuning host-guest interactions through peptide sequence modification.
- The results demonstrated that the modular nature of peptides allows for the systematic exploration of binding properties, offering advantages over traditional methods that rely on simple composition ratios or additives.
Research Impact
- This study establishes a novel screening methodology to identify peptide-based host materials for rare-earth ions, showing a development path for new materials for technological applications.
- The modular nature of peptides offers greater flexibility in material design than conventional methods that rely on composition ratios or additives, enabling fine-tuned control over material properties [8].
- The application of TOF-SIMS for detecting peptide-europium interactions shows how advanced analytical techniques can enable precise characterization of bio-based host materials [6].
Conclusion
The researchers successfully demonstrated a new screening approach for identifying peptide hosts for rare-earth ions using high-density peptide arrays and TOF-SIMS analysis. This work represents an important step towards developing bio-based materials with controlled properties. The combination of peptide array technology with advanced analytical methods enables the efficient screening of thousands of potential host materials in a single experiment, accelerating the discovery and optimization of new bio-inspired materials for applications involving rare-earth ions. Contact us to learn how this technology can assist with your projects.
References
[1] Bonnet, C. S.; Devocelle, M.; Gunnlaugsson, T. Luminescent Lanthanide-Binding Peptides: Sensitising the Excited States of Eu(III) and Tb(III) with a 1,8-Naphthalimide-Based Antenna. Org. Biomol. Chem. 2012, 10, 126-133.
[2] Dikmans, A.; Beutling, U.; Schmeisser, E.; Thiele, S.; Frank, R. SC2: A Novel Process for Manufacturing Multipurpose High-Density Chemical Microarrays. QSAR Comb Sci 2006, 25, 1069-1080.
[3] Fodor, S. P. A.; Read, J. L.; Pirrung, M. C.; Stryer, L.; Lu, A. T.; Solas, D. Light-Directed, Spatially Addressable Parallel Chemical Synthesis. Science 1991, 251, 767-773.
[4] Stadler, V.; Felgenhauer, T.; Beyer, M.; et al. Combinatorial Synthesis of Peptide Arrays with a Laser Printer. Angew Chem Int Ed 2008, 47, 7132-7135.
[5] Madirov, E. Up- and Down- Conversion in Rare Earth-Doped Fluoride Crystalline Materials for Photovoltaic Applications. 2024, Karlsruher Institut für Technologie (KIT)
[6] Muenster, B.; Welle, A.; Ridder, B.; et al. Solid-Material-Based Coupling Efficiency Analyzed with Time-of-Flight Secondary Ion Mass Spectrometry. Appl Surf Sci 2016, 360, 306-314.
[7] Lhoest, J.-B.; Wagner, M. S.; Tidwell, C. D.; Castner, D. G. Characterization of Adsorbed Protein Films by Time of Flight Secondary Ion Mass Spectrometry. J Biomed Mater Res 2001, 57, 432-440.
[8] Zhang, S. Emerging biological materials through molecular self-assembly. Biotechnol Adv 2024, 42, 101234.