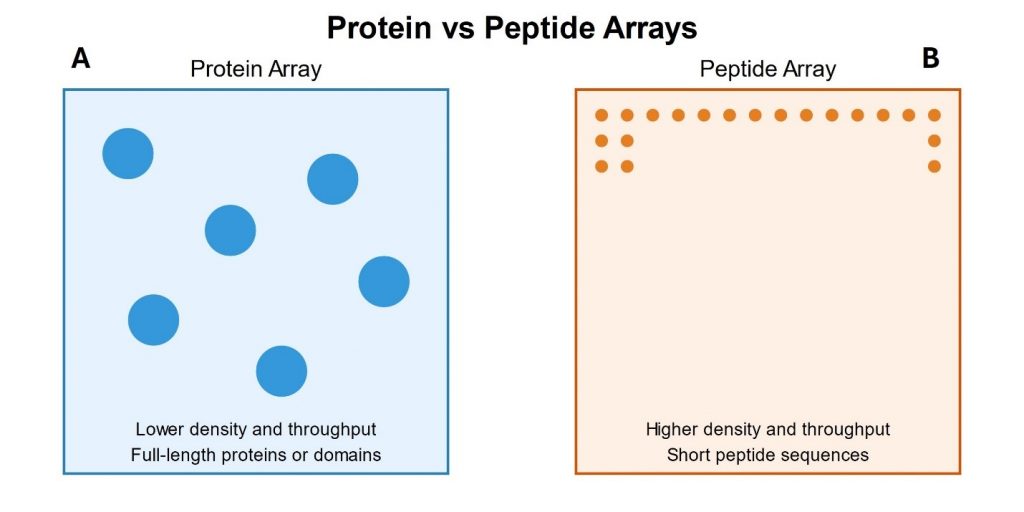
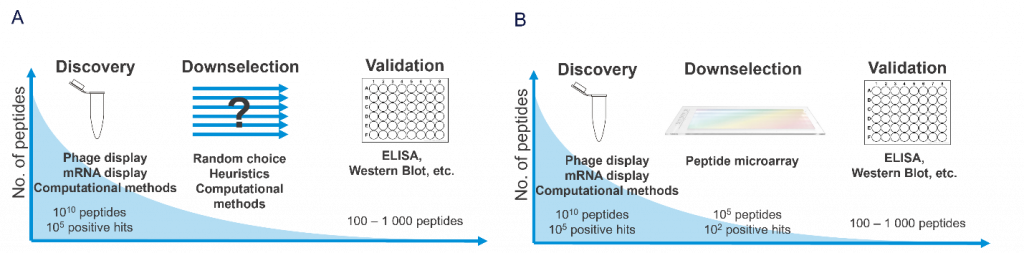
Introduction
Epitope mapping is an important tool for understanding diseases and developing drugs, as it provides comprehensive information on the interactions between antigens and antibodies.
But that’s only half the picture. Epitope mapping has become increasingly important in regulatory and legal affairs: by strengthening intellectual property (IP) protection, proving freedom to operate, and supporting regulatory approval for new biologics and biosimilars.
This blog post briefly explains the concept of epitope mapping, and explores why epitope mapping is vital for IP and regulatory manners, particularly in the development and patenting of protein-based therapeutics.
What is epitope mapping?
Epitope mapping is the process of identifying the specific part of an antigen – the epitope — which an antibody recognizes and binds to. Understanding these binding sites is crucial to characterize antibodies and other protein-based drugs. [1].
What are epitopes?
Epitopes are specific parts of an antigen that are recognized by the immune system. Epitopes can be linear, discontinuous, or conformational in nature.
What are discontinuous epitopes?
Unlike linear epitopes, which consist of a continuous stretch of amino acids, discontinuous epitopes are formed by amino acids that are distant in the primary sequence of the protein but are brought together in their 3D structure (Figure 1).
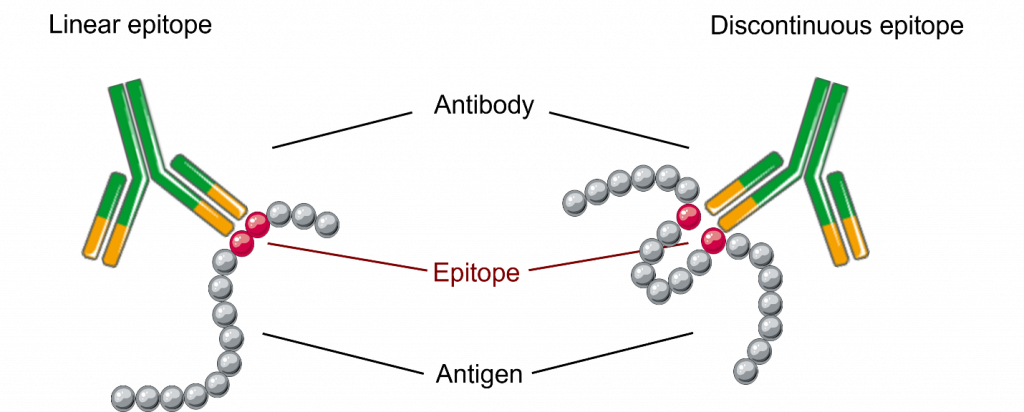
Most epitopes recognized by antibodies are discontinuous. Therefore, understanding discontinuous epitopes is critical to determine antibody specificity and function.
Epitope mapping for Intellectual Property/Patent protection
In line with the evolution of therapeutic antibodies, epitope claims in antibody patents have undergone increasing stringency. Patent offices worldwide, particularly the United States Patent and Trademark Office (USPTO) and the European Patent Office (EPO), now favor structural definitions over functional ones for antibody claims:
Structural definitions describe the physical composition and arrangement of the antibody molecule with its epitope, typically including amino acid sequences of key regions such as complementarity-determining regions (CDRs). In contrast, functional definitions characterize antibodies by what they do rather than what they are made of, focusing on properties like target binding, affinity, or biological effects.
Therefore, by providing structural, detailed, molecular-level information about antibody-antigen interactions, epitope mapping offers a powerful tool for demonstrating the novelty, non-obviousness, and enablement of antibody inventions [3]:
- Enablement and sufficiency of disclosure:
- Provides detailed characterization of the antibody-antigen interaction
- Demonstrates a clear understanding of the invention, supporting claims of reproducibility
- Helps define the scope of functional claims more precisely
- Written description (USPTO):
- Offers specific structural information about the binding site
- Supports correlation between structure and function claims
- Clarity (EPO):
- Allows for more precise claim language, reducing ambiguity
- Helps define the boundaries of the claimed invention
- Novelty:
- Differentiates the claimed antibody from prior art by specifying unique binding characteristics
- Helps identify subtle differences that may not be apparent from sequence data alone
- Non-obviousness/Inventive step:
- Can reveal unexpected binding properties or mechanisms
- Supports arguments for non-obviousness by demonstrating unique epitope recognition
- Identification of discontinuous epitopes generally provides stronger support for the inventive step than linear epitopes. This is because they are more complex and less predictable, hence more difficult to identify and characterize.
- Technical effect (EPO):
- Provides evidence for specific binding properties across the claim scope
- Helps demonstrate that the claimed technical effect is achieved for various antibodies within the genus
- Structural vs. functional claims:
- Bridges the gap between structural and functional descriptions
- Can support broader functional claims by providing a detailed mechanistic understanding
- Post-grant defense:
- Provides additional data to defend patent validity if challenged
Epitope mapping in providing Freedom to Operate (FTO)
Epitope mapping enables researchers to identify whether the drug they develop targets different epitopes from those covered by existing patents. If the epitope is distinct or interacts uniquely with the antigen, it can demonstrate non-infringement of competitor IP.
Conducting epitope mapping early in development aids in the design of products that avoid patent infringement by ensuring distinctiveness. If contested, mapping offers critical evidence of product differences.
Epitope mapping can also be used to discover novel unpatented epitopes. This strategy creates additional barriers for competitors, expands a company’s IP portfolio, and enhances the FTO.
Epitope Mapping in Regulatory Approval for Biologics
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) encourage epitope mapping results in drug applications to better understand immunogenicity and the mechanism of action of drugs.
Epitope mapping helps identify specific regions of an antigen that a drug targets, which is essential for demonstrating the therapeutic effects of the drug. By mapping these interactions, companies can provide detailed evidence of a drug’s mechanism of action, which is a key requirement for regulatory approval.
Moreover, epitope mapping is important for evaluating immunogenicity (potential of a drug to trigger an immune response). Both FDA and EMA mandate comprehensive immunogenicity evaluations to ensure the safety of biologics, as unexpected immune responses can cause adverse effects or reduced efficacy [4, 5].
Epitope Mapping in Regulatory Approval of Biosimilars
Epitope mapping is also important in the process of approving biosimilar products as it shows that the biosimilar targets the same epitopes as the reference biologic.
FDA and EMA demand strong evidence that a biosimilar interacts with the same targets as the original product to ensure therapeutic equivalence. By comparing specific epitopes, epitope mapping confirms that the biosimilar shares a similar mechanism of action with the reference. This process also helps to prevent unexpected immune responses by ensuring the same antigenic targets [6,7].
Conclusion
Epitope mapping provides molecular details that demonstrate novelty and non-obviousness in invention. This type of characterization strengthens patent claims, aids in dispute resolution, guides development, and supports regulatory compliance. Therefore, epitope mapping is a valuable method to help secure intellectual property, operational freedom, and regulatory compliance in modern therapeutic development.
At Axxelera, we can help you map linear, discontinuous and conformational epitopes, all in one experiment, making your epitope mapping projects quick and effortless. Contact us for more details.
References
[1] Van Regenmortel MH. What is a B-cell epitope? Methods Mol Biol. 2009;524:3-20. doi: 10.1007/978-1-59745-450-6_1. PMID: 19377933.
[2] Bogahawaththa D, Chandrapala J, Vasiljevic T. Modulation of milk immunogenicity by thermal processing. Int Dairy J. 2017; 69:23-32. doi: 10.1016/j.idairyj.2017.01.010
[3] Deng X, Storz U, Doranz BJ. Enhancing antibody patent protection using epitope mapping information. MAbs. 2018;10(2):204-209. doi: 10.1080/19420862.2017.1402998.
[4] U.S. Food and Drug Administration (FDA). “Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products.”
[5] European Medicines Agency (EMA). “Guideline on Immunogenicity Assessment of Therapeutic Proteins.”
[6] U.S. Food and Drug Administration (FDA). “Scientific Considerations in Demonstrating Biosimilarity to a Reference Product.”
[7] European Medicines Agency (EMA).” Guideline on Similar Biological Medicinal Products.”